What you need to know
The information in this document was correct at the time of publication. Please ensure you refer to the up-to-date guidance published in the Green Book and the UKHSA Covid-19 vaccination programme collection
COVID-19 vaccination is now a targeted offer for those at higher risk of severe COVID-19 disease during planned seasonal campaigns. These campaigns are currently bi-annual: an autumn and a spring campaign.

The Covid-19 spring vaccination programme commences on 1st April and will run until 17th June 2025.
Eligibility
Only those individuals who are eligible for a dose during any current campaign should receive a dose of vaccine.
The following groups should be offered a COVID-19 vaccine:
- all adults aged 75 years and over including individuals aged 74 who will have their 75th birthday before the campaign ends (17th June 2025). (Please note the age at which other older adults now become eligible has been raised to 75 years)
- residents in a care home for older adults
- individuals aged 6 months and over who are immunosuppressed (as defined in the “immunosuppression” row of table 3 and table 4 of Green Book chapter 14a p32)
Pregnancy
Pregnancy is not an indication for COVID-19 vaccination during the spring 2025 campaign. However, women who are pregnant, planning pregnancy or are in the immediate postpartum period should be offered the vaccine if they are immunosuppressed (as defined in the Green Book). The vaccines can be administered at any stage of pregnancy.
Additional resources for healthcare professionals and for pregnant and breastfeeding women are available from the UKHSA and the Royal College of Obstetricians and Gynaecologists (RCOG).
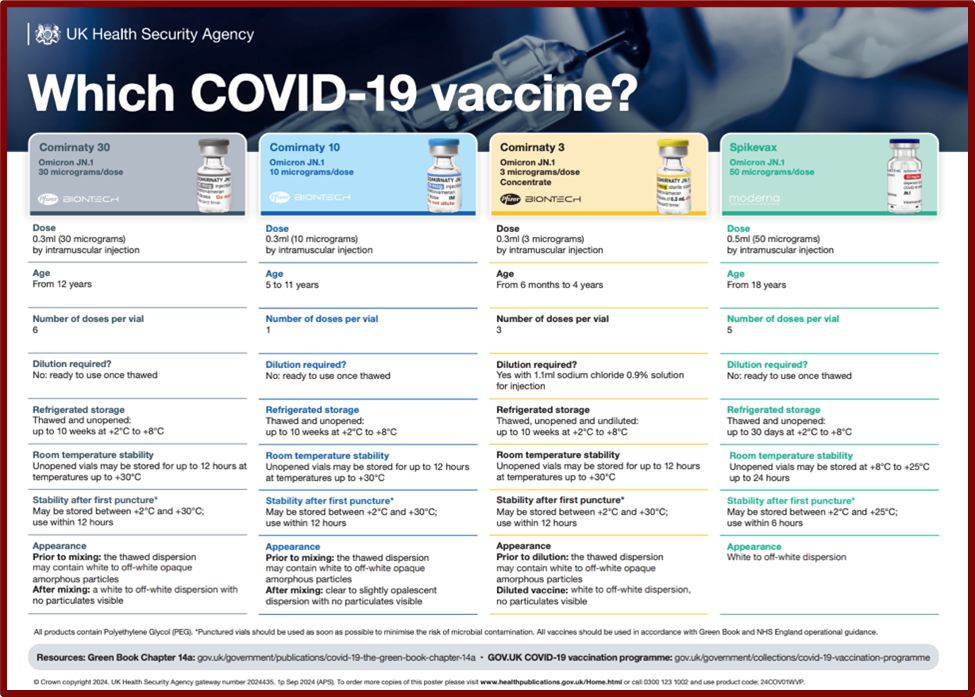
Recommended Vaccines
The recommended vaccines are all monovalent products containing mRNA for the spike protein of the JN.1 sub-lineage of the Omicron variant strain of the SARS-CoV-2 virus.On
Remember that not all vaccines are suitable for every eligible individual; The JCVI recommendations for vaccine type, dose and schedule – which are set out in the Green Book COVID-19 chapter – should at all times be followed.
Eligible adults aged 18 years or over
- a 0.3ml dose of Pfizer BioNTech (Cominarty 30 JN.1) 30 micrograms/dose COVID-19 vaccine
- a 0.5ml dose of Moderna (Spikevax JN.1) 50 micrograms/dose COVID-19 vaccine
Eligible children and young adults aged 12-17 years
- a 0.3ml dose of Pfizer BioNTech (Cominarty 30 JN.1) 30 micrograms/dose COVID-19 vaccine
Eligible children aged 5 to 11 years
- a 0.3ml dose of Pfizer BioNTech 10 (Cominarty 10 JN.1) micrograms/dose
Eligible children aged 6 months to 4 years
- a 0.3ml dose of Pfizer BioNTech 10 (Cominarty 10 JN.1) micrograms/dose
The vaccines have different coloured flip-off caps and packaging colour schemes to help differentiate between them. However, it should never be solely relied upon.
Every member of staff has an important role to play in patient safety.
It is important that before administering any dose of vaccine you are certain that you have selected the correct vaccine product, that it has been correctly stored and prepared and is the correct dose for that individual
The UKHSA Poster Which Covid-19 vaccine poster ? is available as a resource to check the appropriate vaccine
‘Missed doses’
Doses are no longer categorised as ‘primary’ or ‘booster’. All eligible individuals require a single dose of vaccine during the seasonal campaign. Ideally, subsequent doses should be offered around 6 months from any previous dose but, operationally, can be given a minimum of 3 months from any previous dose.
Individuals who have not taken up the offer in previous campaigns therefore cannot be ‘caught-up’ as the dose(s) they missed were intended to protect them during a period of time that has now elapsed. If eligible they should be vaccinated during the current campaign and encouraged to take up any future offers that apply.
Co-administration of vaccines: Older Adults
Some data shows that co-administration of COVID 19 vaccination and RSV vaccination may reduce the immune response to the RSV vaccine. It is therefore recommended that these vaccines should not routinely be scheduled to be given on the same day to older adults. No specific interval is required between administering the vaccines. If it is thought that the individual is unlikely to return for a second appointment or immediate protection is necessary, the vaccines can be administered at the same time. See Green book for further details
Storage
The NHS Specialist Pharmacy Service (SPS) has produced comprehensive guidance and a suite of standard operating procedures (SOPs) relating to all aspects of COVID-19 vaccine use, including storage and transportation, to which staff should refer.

Ordering
COVID-19 mRNA vaccines are centrally procured and can be ordered via either the ImmForm platform or the Foundry system; each provider will have been advised of the appropriate route for them.
Legal Governance

All vaccines are Prescription only Medications (POMS’s). Please ensure you have the appropriate legal governance in place to administer the vaccines.
All the vaccines being supplied for adults and young people are included in the national templates PGD and National Protocols. Patient Group Direction and are available from NHS England and the National Protocol is also available National protocol for COVID-19 vaccine (5 years and over). Individual members of staff must be locally authorised by name to work under these documents.
Written materials for patients

A leaflet ‘A Guide to the Spring 2025 Covid-19 Vaccination’ can be ordered in multiple languages and other formats such as braille, Audio and Large print from the Health Publications website.
The Patient Information Leaflet (PIL) will also be provided with each vaccine pack. Additional copies can be printed from the EMC Website under the individual vaccine details.
Remember that in order to allow informed consent, accessible information must be made available to each individual (or their parent or carer) before they receive the vaccine.
Patient vaccination record cards and stickers should be ordered directly from the Health Publications website.

Training requirements – Covid-19 Vaccination workforce.

Practitioners should follow the UKHSA Covid Vaccinator training recommendations and complete the comprehensive e-learning programme (published on the e-Learning for Health platform).
The e-learning modules include the Core Knowledge session, and this must be completed by any staff member new to Covid-19 vaccination. Experienced Covid-19 vaccinators may want to a revisit the module to refresh their knowledge. Further modules and accompanying assessments should be completed for any vaccine you will be administering as part of the Spring programme.
All staff should check and comply with their commissioner’s/employer’s training and assessment requirements for the provision of a COVID-19 vaccination service.

Further Useful Resources
- Green Book COVID-19 chapter
- Green Book Consent chapter
- UKHSA Coronavirus vaccination programme resources
- Electronic medicines compendium
- e-learning for healthcare (elfh) COVID-19 vaccination sessions
- SPS COVID-19 resources
- Pfizer BioNTech information and education about Comirnaty vaccines for healthcare professionals
- Moderna has produced videos and other resources for their COVID-19 vaccines