This blog provides practitioners with information on the Hepatitis B selective neonatal immunisation pathway, DBS testing at 12 – 18 months and useful resources.
The World Health Organization (WHO) has classified hepatitis as an international public health challenge comparable to malaria, HIV and tuberculosis.
The introduction of the hexavalent vaccine, as part of the universal childhood immunisation programme in 2017, provided an opportunity for collaborative working by the infectious diseases in pregnancy screening programme and national immunisation team. The aim was to develop a quality improvement strategy to improve the care for women living with hepatitis B and their babies.
Even though we are classified as a low prevalence country for Hepatitis B we must continue to strive for optimum delivery of equitable care for all to address inequalities (UKHSA 2023).
Hepatitis B
Hepatitis B is an infectious disease caused by the hepatitis B virus (HBV) that affects the liver. The virus causes both acute and chronic infections. An estimated 257 million people or 3.5% of the global population are living with chronic hepatitis B virus infection: WHO Global hepatitis report 2017.
To learn more about Hepatitis B further information can be found on the NHS health website and Vaccine Knowledge Project
Transmission
Globally, perinatal transmission vertically (from mother to baby) is the most common route of HBV acquisition and represents an important contribution to establishing chronic infections within populations. Hepatitis B is more infectious than other blood borne viruses like Hepatitis C and HIV.
Perinatal transmission rates, in the absence of immunisation of the newborn at birth, can be as high as 90% from higher infectivity mothers and approximately 10 to 40% from lower infectivity mothers. Of those babies who are infected at birth or during the first year of life, around 90% will go on to develop chronic infection. The disease will progress to liver cirrhosis and liver cancer in 15% to 40% of children with chronic infection.
The aim of the UKHSA selective hepatitis B immunisation programme is to prevent babies acquiring Hepatitis B following exposure to their mothers’ blood and body fluids especially around the time of birth. As this is a post-exposure vaccination programme, timely administration of all doses of vaccine (±HBIG at birth) is vital in preventing the baby becoming persistently infected with hepatitis B virus.
The UK is a very low prevalence country however each year in the UK, around 3,000 babies are born to women who have hepatitis B infection. Prevalence of HBV infection is higher in those born in high-endemicity countries, many of whom will have acquired infection at birth or in early childhood.
In pregnant women who are positive for hepatitis B virus, the aim is to eliminate vertical transmission to the baby through timely immunisation with a hepatitis B vaccine. Babies born to mothers who have screened positive for HBV in pregnancy, or whose mothers have acute hepatitis B infection in pregnancy, are offered an accelerated course of hepatitis B immunisation starting at birth and continuing as part of the routine childhood immunisation programme with hexavalent vaccine.
Ensuring Optimal Care for Infants Born to Hepatitis B Positive Mothers: A Guide for Practice Nurses and other immunisation practitioners
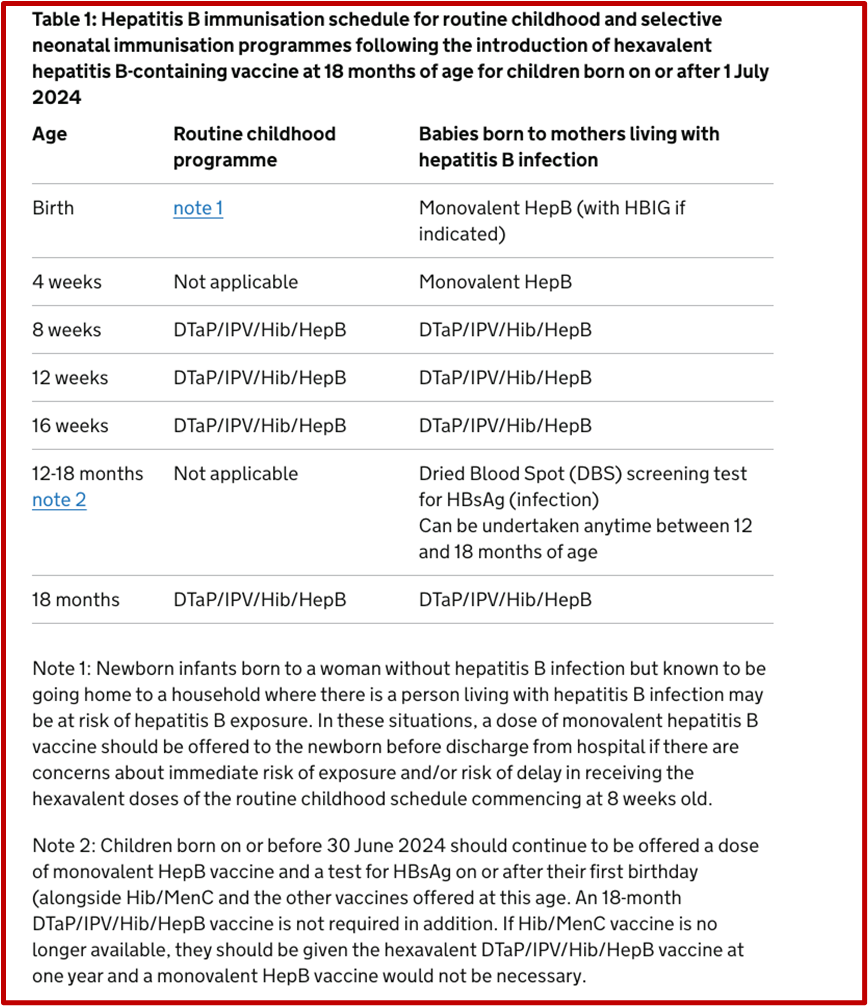
Hepatitis B Vaccination Schedule
For infants born after 1st July 2024
Infants born to hepatitis B positive mothers are at a heightened risk of contracting the virus during childbirth. To mitigate this risk, a structured vaccination schedule is essential:
💉 At birth: Monovalent hepatitis B vaccine ideally within 24 hours of birth. Babies born to pregnant women who have high infectivity risk should also receive HBIG.
💉 4 weeks of age: Administer second dose of the monovalent hepatitis B vaccine.
💉 8, 12, and 16 weeks of age: Administer the routine childhood immunisations, which include the hexavalent vaccine (DTaP/IPV/Hib/HepB).
💉 12 months of age: Administer a booster dose of the hexavalent vaccine (DTaP/IPV/Hib/HepB)
12 – 18 months of age: Test for HBsAG (Dried blood spot test)
Adherence to this schedule is crucial, as completing the full course significantly reduces the risk of the infant developing chronic hepatitis B infection.
The table below (right hand column) clarifies the selective neonatal immunisation pathway
Dried Blood Spot (DBS) Testing at 12 – 18 Months
As chronic infection in an infant is asymptomatic, testing between 12 – 18 month is strongly recommended. It’s imperative to assess the infant’s hepatitis B status to ensure that vaccination has been effective, and that the child has not contracted the virus.
A free national dried blood spot (DBS) testing service for babies is offered by UKHSA to improve ease and uptake of testing, particularly in primary care. The DBS test has been validated for detecting hepatitis B surface antigen (HBsAg) which is the marker for infection.
The DBS test is a minimally invasive method suitable for primary care settings:
Procedure: A simple heel-prick is performed to collect a few drops of blood on a filter paper card.
Advantages:
✅ Simplicity and Convenience: The test can be conducted alongside the 12-month booster vaccination, reducing the need for additional appointments however can be also undertaken anytime between 12 – 18 months either opportunistically or via a booked appointment.
✅ Minimally Invasive: The heel-prick method is less distressing for infants compared to venous blood sampling.
✅ Accessibility: Facilitates testing in primary care settings, eliminating the need for hospital referrals.
Refer to the UKHSA leaflet: How to take a dried blood spot sample

If the national DBS service is not used the infant should be referred to paediatric phlebotomy services ensuring the child is tested for infection.
For further information please refer to the UKHSA guidance document Hepatitis B dried blood spot (DBS) testing for children.
For infants born on or before 30th June 2024
Children born on or before 30 June 2024 should continue to be offered a dose of monovalent HepB vaccine and a test for HBsAg on or after their first birthday (alongside Hib/MenC and the other vaccines offered at this age. An 18-month DTaP/IPV/Hib/HepB vaccine is not required). Refer to Green Book Hepatitis B chapter 18
Your role as an Immunisation practitioner
📚 Education and Communication: Inform parents about the importance of adhering to the vaccination schedule and the necessity of the 12- 18 month DBS test.
⏰ Timely Administration: Ensure vaccines are administered according to the recommended timeline.
🩸 Sample Collection: Be proficient in performing DBS sample collection and ensure timely dispatch to the designated laboratory.
🗂️ Follow-Up: Monitor and communicate test results to parents and coordinate any necessary referrals for infants who test positive.
By diligently following these guidelines and collaborating closely with parents and healthcare teams, you contribute significantly to the prevention of hepatitis B transmission and the promotion of long-term health in these vulnerable infants.
Resources for Healthcare professionals

Hepatitis B vaccine for at-risk infants aide memoire
This is a guidance document for healthcare professionals (mainly in primary care) on the hepatitis B vaccination schedule and testing for babies born to women living with hepatitis B. It acts as a reminder of the vaccination doses and testing schedule for the child.
For those providers of the Neonatal Hepatitis B Pathway, a video has been produced by NHS Northeast and Yorkshire, which highlights the importance of the DBS test, for infants born to mothers living with Hepatitis B. It also includes a physical demonstration of the technique. It is recommended you watch this video.
🔗 Hepatitis B: antenatal screening and selective neonatal immunisation pathway
🔗 Hepatitis B dried blood spot (DBS) testing for infants
🔗 Green Book chapter 18 on hepatitis B
🔗 Hexavalent combination vaccine: programme guidance
Resources including guidance, leaflets and educational materials are available to support professionals in the delivery of the hepatitis B screening and immunisation pathways. The health publications website has links to all the resources is available free to order or download. https://find-public-health-resources.service.gov.uk/search
Resources for parents
Leaflets for parents are available from the health publications website link:
Leaflets for parents are available from the health publications website link: Hepatitis B: screening care in pregnancy and protecting your baby – GOV.UK

